Six scientists analyzed the article and estimated its overall scientific credibility to be ‘very low’. more about the credibility rating
A majority of reviewers tagged the article as: Biased, Cherry-picking, Inaccurate, Misleading.
SCIENTISTS’ FEEDBACK
SUMMARY
Research shows that the pH of the ocean is currently decreasing due to human emissions of CO2 and that this already has negative consequences for marine ecosystems—and continued emissions is expected to have further negative consequences. The article in The Spectator claims the contrary. The scientists who have analyzed the article show that it contains significant inaccuracies, notably for its core assumptions, and misrepresents scientific studies and scientists it cites to make its point.
Reviewers also note that the article knocks down strawman arguments that do not represent the state of scientific knowledge (scientists do not claim the ocean will become a “giant acid bath”) and uses derogatory language by referring to ocean acidification researchers as “alarmists”.
Note: This article, published in April 2016, is being assessed now because of renewed interest in the media following IPSO’s ruling (IPSO is the UK Independent Press Standards Organisation).
See all the scientists’ annotations in context
REVIEWERS’ OVERALL FEEDBACK
These comments are the overall opinion of scientists on the article, they are substantiated by their knowledge in the field and by the content of the analysis in the annotations on the article.
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
As Phil Williamson has carefully documented, it is a story built upon selective back-grounding, based upon dubious sources, and presented in support of the author’s own predetermined storyline and conclusion.
Adam Subhas, PhD candidate, Caltech:
This article bases its arguments on non-peer-reviewed publications and a study which uses poorly calibrated historical data. The article also oversimplifies the science behind ocean acidification in an attempt to trivialize well-understood chemical principles that predict how CO2 dissolving into the ocean will affect ocean pH.
Ken Caldeira, Senior Scientist, Carnegie Institution for Science:
The chemistry of ocean acidification is well understood. Negative biological consequences have been documented for many marine organisms in a diverse set of carefully controlled experiments.
I was involved in an experiment in Australia’s Great Barrier Reef where we added an ‘antacid’ to a plume of seawater, bringing seawater chemistry closer to what it was several hundred years ago. We let that water flow over a patch of reef and measured an increase in the growth rate of the reef. This showed that the increase in acidity caused by our CO2 emissions is already slowing reef growth, harming the reef. Our measurements come out of sophisticated and carefully calibrated scientific instruments. They measure what is out there in the physical world, without any reference to our political views or our degree of alarm. Our measurements are not a matter of opinion.
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
This is an appalling article which distorts the scientific evidence by disqualifying thousands of peer-reviewed articles and highlighting only two un-peer-reviewed publications from non-expert authors.
Tullio Rossi, Marine biologist, University of Adelaide:
This is by far the most inaccurate article on ocean acidification I have ever seen. It is an explosive mix of false statements, cherry picking, and plain anti-science feelings.
Notes:
[1] See the rating guidelines used for article evaluations.
[2] Each evaluation is independent. Scientists’ comments are all published at the same time.
KEY TAKE-AWAYS
The statements quoted below are from the article; comments and replies are from the reviewers.
“In 2004, two NOAA scientists, Richard Feely and Christopher Sabine, produced a chart showing a strong correlation between rising atmospheric CO2 levels and falling oceanic pH levels. But then, just over a year ago, Mike Wallace, a hydrologist with 30 years’ experience, noticed while researching his PhD that they had omitted some key information[…] his results were surprising: there has been no reduction in oceanic pH levels in the last -century.”
Tullio Rossi, Marine biologist, University of Adelaide:
This is plain wrong. Oceanic pH levels decreased by 0.1 units compared to pre-industrial levels. This corresponds to a 30% increase in acidity. For a primer on pH, see this NOAA explainer.
Peer-reviewed references:
- Doney et al (2009) Ocean acidification: the other CO2 problem, Annual Review of Marine Science
- Raven et al (2005) Ocean acidification due to increasing atmospheric carbon dioxide, The Royal Society
Ken Caldeira, Senior Scientist, Carnegie Institution for Science:
This statement just flies in both the face of observational facts and a basic understanding of chemistry. I would love to hear a cogent explanation of how atmospheric CO2 levels could rise over the course of a century without producing a decrease in ocean pH.
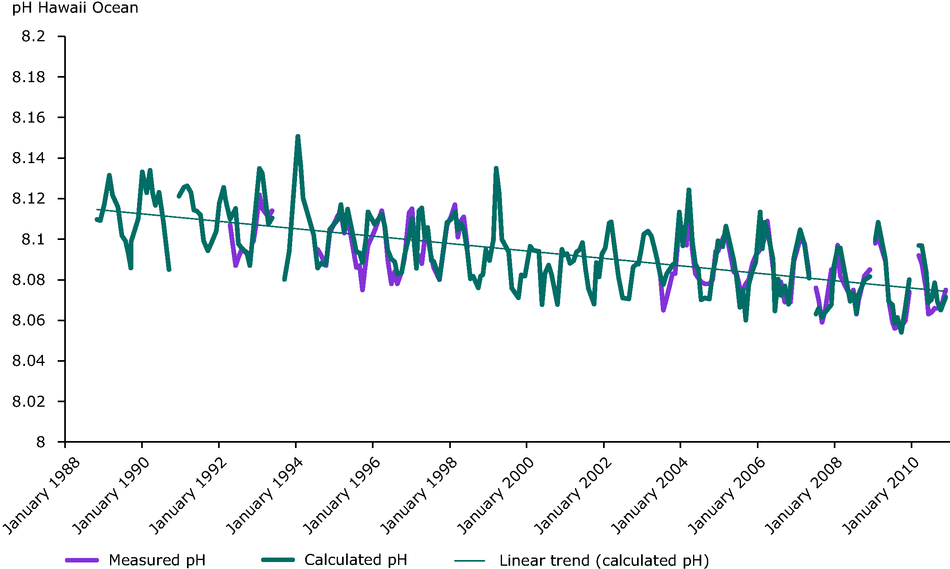
Observations near Hawaii and several other open ocean environments show clear decreasing trends in ocean pH. See, for example this report from the European Environment Agency.
It is one thing to challenge future projections, but rejecting well-established scientific facts is another thing entirely.
Figure: Decline in pH measured at the Aloha station as part of the Hawaii Ocean time-series. Source
Richard Feely, Senior Scientist, NOAA's Pacific Marine Environmental Laboratory:
Michael Wallace contacted Chris Sabine and myself several years ago and asked how to get access to historical pH measurements in the oceans so he could determine long-term trends of global ocean pH for himself. We directed him to both modern (as published in Feely et al., 2008) and historical pH measurements archived at the NOAA National Centers for Environmental Information. We cautioned him that the earlier data sets prior to 1989 had significant issues with data quality as described in the document that went along with the data set obtained from NCEI. Mr. Wallace went on to perform his analysis of the historical data without regard for the oceanographic community’s concerns about the data quality or for the proper methodology to perform this kind of analysis. He chose to publish his results within the context of an interview written by Marita Noon in the Farmington Daily Times. We responded by correcting the record by formally restating our concerns about Mr. Wallace’s incorrect use of the historical data on our website. In short, Wallace’s chart does not show any kind of useful trend in global ocean pH because the data he used, and the way he used them, were not appropriate for this kind of analysis. In the first place, the pH measurements prior to 1989 were not reliable enough to detect small pH changes over that period. In addition, companion meta data on sensor calibration, pH scales, and temperature corrections were not available. Finally, the data were so limited that no meaningful global averages could be determined. The article by Mr. Delingpole in The Spectator failed to address these important issues, as Philip Williamson correctly points out in his response to the article.
“Ocean acidification is the terrifying threat whereby all that man-made CO2 we’ve been pumping into the atmosphere may react with the sea to form a sort of giant acid bath.”
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
That is incorrect. No peer-reviewed article claim that the ocean will become acid (pH < 7).
“‘Acid’ was chosen, Moore believes, because it has ‘strong negative connotations for most people’.”
Tullio Rossi, Marine biologist, University of Adelaide:
The terms acidification was not chosen for its negative connotation but rather because it defines the direction of change. Here is a simple example: when you describe the cooling of your coffee what term do you use? Cooling or un-warming? If the temperature is going down we say it is cooling. If the temperature is going up we say it is warming. The same applies to acidity. When the pH of something goes down we say that it is acidifying. That simple.
“so more correctly it should be stated that the seas are becoming slightly less alkaline.”
Adam Subhas, PhD candidate, Caltech:
This is actually not correct. Invasion of CO2 into the ocean does not change its alkalinity at all. By adding carbonic acid, and keeping alkalinity constant (defined as either the difference between dissolved cations and anions, or the excess of acid-base species at the CO2 equivalence point), the pH decreases.
“seawater has a large buffering capacity which prevents dramatic shifts in pH;”
Adam Subhas, PhD candidate, Caltech:
This is true; however, the amount of CO2 emitted by humans is also massive, a large portion of which has already been absorbed by the oceans. Since pH is relatively insensitive to changes in CO2, it is often not the right parameter to look at in the whole system. For example, over the range of pH values which the author states are found in natural seawater, the surface pCO2 changes from 180 ppm (pH 8.3) to 1500 ppm (pH 7.5). Thus, a mean change in ocean pH of 0.3 pH units represents almost a tripling of seawater pCO2.
“[…]The impact on calcification, metabolism, growth, fertility and survival of calcifying marine species when pH is lowered up to 0.3 units […] is beneficial, not damaging. Marine life has nothing whatsoever to fear from ocean acidification.”
Ken Caldeira, Senior Scientist, Carnegie Institution for Science:
There is much evidence available to falsify this statement. Many experiments have shown substantial negative biological responses at these levels of pH change. Of course, some organisms are relatively unaffected by these levels.
An older review that is available here without a paywall:
- Langdon (2002) Review of experimental evidence for effects of CO2 on calcification of reef builders. In Proc. 9th Int. Coral Reef Sym.
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:“Then it will destroy all the species that depend on it — causing an almighty mass extinction which will wipe out the fishing industry and turn our oceans into a barren zone of death.”
That is not a truthful summary of the scientific literature. Check, for example, the meta-analysis of Kroeker et al. (2013) who describe processes and organisms that do not seem to be affected by ocean acidification.
- Kroeker et al. (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology.
“a killer analysis conducted by Craig Idso of all the studies which have been done on the effects of reduced pH levels on marine life.”
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
This publication is not peer-reviewed, cherry-picks articles and does not involve proper statistical testing. It does not, therefore, qualify as a “killer analysis”! The comprehensive metanalysis that was performed by Kroeker et al. (2013) revealed decreased survival, calcification, growth, development and abundance in response to acidification when the broad range of marine organisms is pooled together. However, the magnitude of these responses varies among taxonomic groups.
- Kroeker et al (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology.
Richard Feely, Senior Scientist, NOAA's Pacific Marine Environmental Laboratory:
The Idso meta-analysis described by Delingpole was never published. It does not take into account the proper method of proportional scaling analysis. It does not demonstrate how negative effects will impact ecosystem services and food-web processes that can have an effect on economically important fish and shellfish. It does not address the impacts we are already seeing on important fish food, such as pteropods (see Bednarsek et al., 2012, 2014; Feely et al., 2016). There are several highly credible published meta-analysis studies (Kroeker et al. ,2013; Wittman & Pörtner, 2013; and Busch and McElhany, 2016) that have told a much different story than Idso’s unpublished work. Delingpole failed to even mention these other studies, which show very significant impacts on several marine taxa. In summary, Delingpole’s article demonstrates a complete lack of appreciation of scientific literature on this topic and the proper choice of scientific methods for data analysis and synthesis that leads to a more accurate understanding of the present-day and future impacts of ocean acidification.
- Bednaršek et al (2012) Extensive dissolution of live pteropods in the Southern Ocean, Nature Geoscience
- Bednaršek et al (2014) Limacina helicina shell dissolution as an indicator of declining habitat, Proc. of the Royal Society B
- Feely et al (2016) Chemical and biological impacts of ocean acidification along the west coast of North America, Estuarine, Coastal and Shelf Science
“First, marine species that calcify have survived through millions of years when CO2 was at much higher levels; second, they are more than capable of adapting — even in the short term — to environmental change”
Ken Caldeira, Senior Scientist, Carnegie Institution for Science:
Many marine organisms respond to changes in calcium carbonate mineral saturation states. These depend not only on pH, but also on factors including amounts of carbon and calcium in the ocean. One of the most important factors is ocean alkalinity, which varies on time scale of many thousands of years. In the geologic past, when atmospheric CO2 was high, ocean alkalinity was also high, and so carbonate mineral saturation states could remain high.
Unfortunately, on the timescale of centuries or decades, changes come too fast for the ocean’s natural processes to buffer ocean carbonate mineral saturation states.
Directly comparing effects of high CO2 levels that developed in the geologic past over many millions of years with high CO2 levels developing today over decades and centuries shows a fundamental lack of understanding of well-established global geochemical cycles. This open access article explains some of the relevant chemistry:
- Cao et al (2016) Simulated effect of deep-sea sedimentation and terrestrial weathering on projections of ocean acidification, Journal of Geophysical Research Oceans
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
Some calcifying species were indeed abundant in the Cretaceous, a time at which the atmospheric CO2 concentration was high. However, seawater alkalinity was also high due to intense weathering on land. Hence, the concentration of carbonate ions (CO3, which controls calcification) was elevated. That compensation does not happen today and will not happen in the near future because total alkalinity does not change significantly on time scales of centuries. There is ample evidence in the literature for that.
Some fast-growing species are indeed able to develop some level of adaptation after several hundreds of generation. Overall, there is evidence that all past episodes of ocean warming, acidification and deoxygenation have led to mass extinctions. Furthermore, there is no calcifier close to CO2 vents, suggesting that adaptation has limited capabilities.
“if oceans do become warmer due to ‘climate change’, the effect will be for them to ‘outgas’ CO2, not absorb more of it”
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
Incorrect: The effect of warming is completely overwhelmed by the effect of increased atmospheric CO2. Hence, the ocean will continue to absorb massive amounts of CO2 in the future, despite ocean warming.
“[ocean acidification was ] First referenced in a peer-reviewed study in Nature in 2003”
Jean-Pierre Gattuso, Research Professor, CNRS, Université Pierre et Marie Curie and IDDRI:
The expression “ocean acidification” was actually introduced in 2001 by Broecker and Clark*. But the chemical processes involved have been know for a very long time at least the 1950s) and the impact of low pH (elevated acidity) on marine organisms since the early 1900s. The earliest experiments even predate the definition of pH by Sørensen in 1909.
- Broecker & Clark (2001) A dramatic Atlantic dissolution event at the onset of the last glaciation, Geochemistry Geophysics Geosystems
“Howard Browman, a marine scientist for 35 years, has published a review in the ICES Journal of Marine Science of all the papers published on the subject. His verdict could hardly be more damning. The methodology used by the studies was often flawed; contrary studies suggesting that ocean acidification wasn’t a threat had sometimes had difficulty finding a publisher. There was, he said, an ‘inherent bias’ in scientific journals which predisposed them to publish ‘doom and gloom stories’.”
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
The following decomposition of this excerpt from Mr. Delingpole’s article in The Spectator identifies inaccuracies in his reporting that lead to misrepresentation of the content and intent of my article. Importantly, Mr. Delingpole never contacted me to verify that his reporting on my article was accurate. Nor was I contacted by the UK’s Independent Press Standards Organisation during their investigation of the accuracy of Mr. Delingpole’s article in The Spectator.
“Howard Browman, a marine scientist for 35 years, has published a review in the ICES Journal of Marine Science of all the papers published on the subject.”
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
The article that Mr. Delingpole is referring to is not “a review”, but an introduction to a special theme issue on the topic of ocean acidification[…] The introduction does not review all of the nearly 4000 articles on the subject. Rather, it presents an overview of the sub-set of research dealing with biological/ecological effects of ocean acidification.
“His verdict could hardly be more damning.
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
On p. 530 of the introduction, I state: “Although I call for a more sceptical scrutiny and balanced interpretation of the body of research on OA [Ocean Acidification], it must be emphasized that OA is happening and it will have effects on some marine organisms and ecosystem processes.” This is hardly a verdict that “…could hardly be more damning.”
“The methodology used by the studies was often flawed”
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
My introduction does not present a quantitative assessment of the frequency of occurrence of methodological flaws in the ocean acidification literature.
“There was, he said, an ‘inherent bias’ in scientific journals which predisposed them to publish ‘doom and gloom stories’.”
Howard Browman, Principal Research Scientist, Norwegian Institute of Marine Research:
The words “inherent bias” do not appear in my article. Rather, I refer to “publication bias”. In research, an “inherent bias” is one which is inextricably tied to the core nature of the phenomenon being studied and cannot, therefore, be eliminated by increasing the sample size or choosing a different estimator. “Publication bias”, on the other hand, refers to the general (across all of science) phenomenon by which studies presenting positive results – supporting the hypothesis being tested – are more likely to be published than those reporting negative results. This sometimes creates a situation where published studies may be systematically different from unpublished studies – for example, studies showing an impact of ocean acidification might be published more easily and in higher profile journals than studies showing no impact. However, I only allude to this possibility in the introduction; I do not asses it quantitatively (something that would, in fact, be very difficult to do). Finally, the words “doom and gloom” do not appear in my introduction.